note d'application sur l'eau ultrapure pour l'analyse HPLC
note d'application sur l'importance de l'eau ultrapure en HPLC

Contenu du document
introduction
HPLC est une procédure analytique pour la séparation, l'identification et la quantification de substances utilisant la chromatographie liquide. Les débuts de la HPLC - chromatographie liquide à haute pression - remontent aux années 60. Grâce à l'amélioration des matériaux de colonne et des équipements, elle est connue sous le nom de chromatographie liquide à haute performance depuis la fin des années 70.
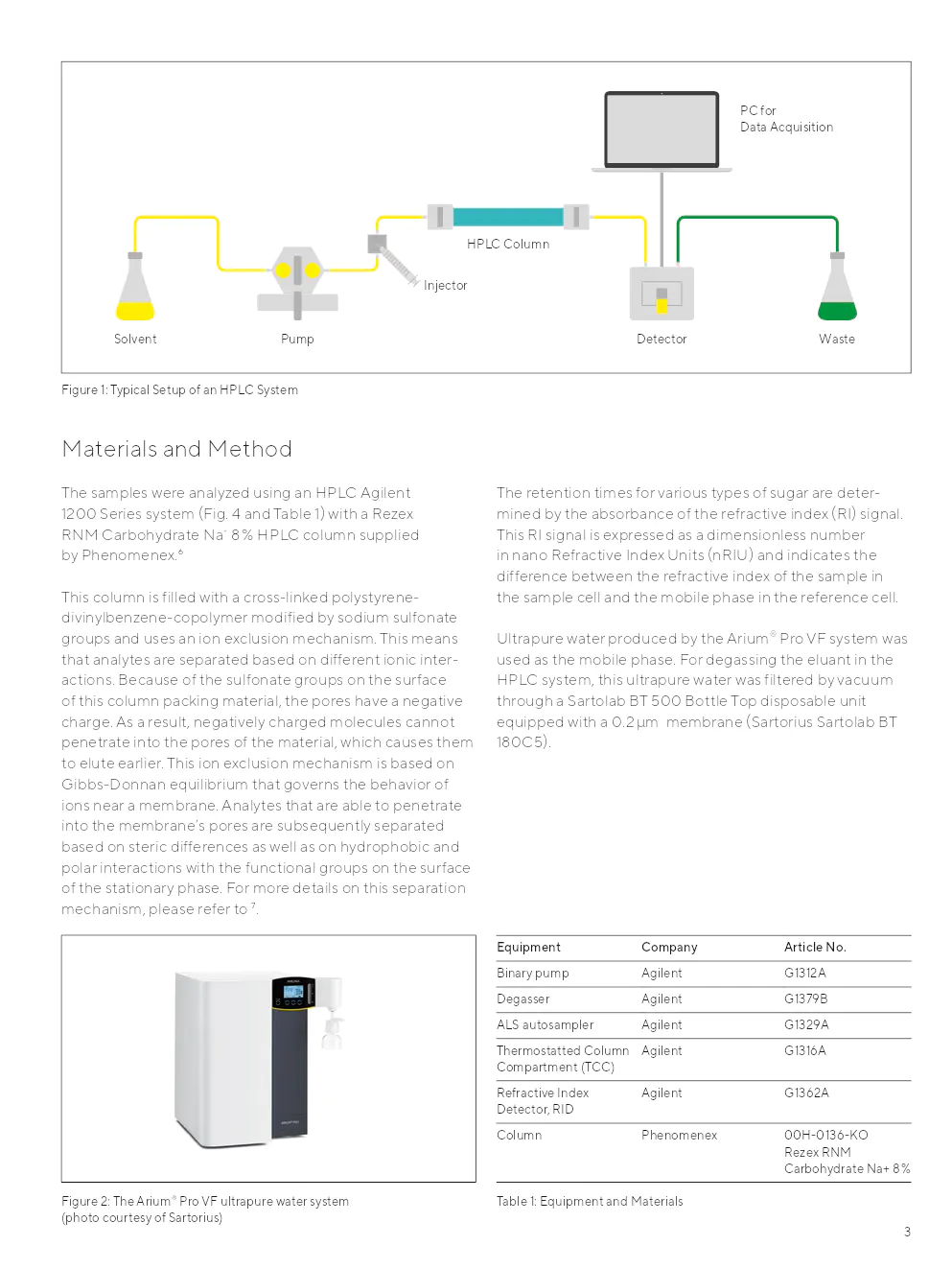
En HPLC, le mélange à séparer est transféré sur une colonne avec un solvant (agent d'élution) ou un mélange de solvants (éluant | phase mobile) par un injecteur et une pompe. La colonne est un tube, dans la plupart des cas en acier inoxydable, rempli de ce que l'on appelle la phase stationnaire. La phase stationnaire consiste généralement en gel de silice poreux ou en particules polymères avec des ligands chimiques liés à leur surface. Ces ligands sont responsables des interactions sélectives entre les analytes et la phase stationnaire, nécessaires pour une séparation chromatographique efficace.
employing arium® pro vf to purify water for use as an eluant
Parmi les applications typiques pour la HPLC figure l'analyse du sucre. Des tests ont été réalisés pour caractériser la qualité des membranes. D'une part, les membranes ont été testées pour leur capacité à éliminer les molécules de sucre et, d'autre part, l'activité des membranes immobilisées par enzyme a été déterminée. À cette fin, des sucres tels que le raffinose, le glucose et le fructose ont été dosés.
Dans les analyses avancées, le sucre est souvent dosé par chromatographie en couche mince (TLC), chromatographie en phase gazeuse (GC) et chromatographie liquide à haute performance (HPLC). Ces méthodes sont utilisées lorsque les mélanges contenant plusieurs types de sucre doivent être séparés.
description of the arium® pro vf ultrapure water system
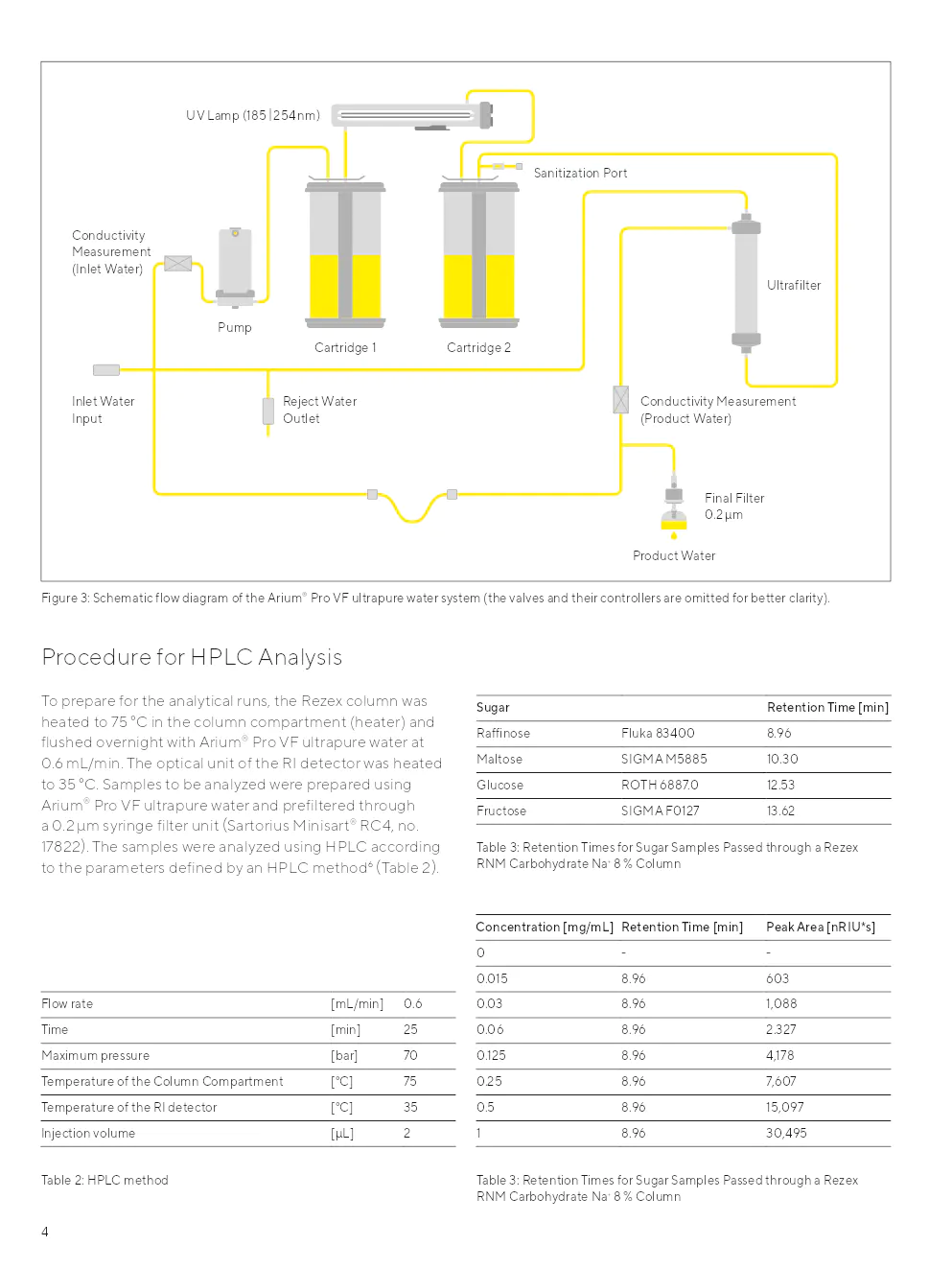
Le système arium® pro vf est conçu pour produire de l'eau ultrapure à partir d'eau potable prétraitée en éliminant les contaminants qui sont encore présents dans cette eau potable. La production d'eau ultrapure nécessite une recirculation continue et un débit d'eau constant, réalisé à l'aide d'un système de pompe intégré avec pression contrôlée. La conductivité de l'eau est mesurée à l'entrée de l'eau d'alimentation et directement au niveau de la sortie (eau de produit).
matériel et méthode
Les échantillons ont été analysés en utilisant un système HPLC Agilent 1200 Series avec une colonne HPLC Rezex RNM Carbohydrate Na+ 8 % fournie par Phenomenex. Cette colonne est remplie d'un copolymère de polystyrène-divinylbenzène réticulé modifié par des groupes sulfonates de sodium et utilise un mécanisme d'exclusion ionique.
procedure for HPLC analysis
Pour préparer les analyses, la colonne Rezex a été chauffée à 75 °C et rincée toute la nuit avec de l'eau ultrapure arium® pro vf à un débit de 0.6 mL/min. Les échantillons préparés avec l'eau ultrapure arium® pro vf ont été analysés conformément aux paramètres définis par une méthode HPLC.
conclusion
Les résultats montrent que l'eau ultrapure produite par arium® pro vf peut être utilisée comme phase mobile pour l'analyse HPLC des saccharides solubles dans l'eau décrits dans cet article. L'eau ultrapure produit une conductivité de 0.055 µS/cm peut être considérée comme pratiquement exempte de contaminants. Arium® pro vf offre une alternative abordable à l'eau ultrapure vendue commercialement pour préparer des éluants hautement purs pour l'analyse HPLC.
Entreprises concernées :
Document protégé
Document uniquement accessible aux visiteurs connectés
Pas encore de compte ?
Inscrivez-vous
Déjà un compte ? Cliquez ici pour vous connecter
Connectez-vous