note d'application sur l'importance de mesurer l'humidité des résines plastiques dans les dispositifs médicaux
note d'application sur la mesure de l'humidité des résines plastiques pour les dispositifs médicaux

Contenu du document
Date : octobre 2019
Mots-clés :
- Omnimark
- Mark 3
- Analyseur d'humidité pour dispositifs médicaux
- Analyse de l'humidité des plastiques
- Norme ASTM sur l'humidité des résines plastiques
- Test de l'humidité des résines plastiques
Auteurs :
King Snyder, Dennis Acord (USA) et Evelyn Marschall (Allemagne)
Résumé :
Le contenu en humidité est une variable essentielle à surveiller dans la production de pièces de dispositifs médicaux en plastique. La norme ASTM D6869 recommande l'utilisation de la titration par Karl Fischer (KF) pour mesurer l'humidité, bien que coûteuse et nécessitant des produits chimiques toxiques, limitant son utilisation à des chimistes formés. L'analyseur d'humidité par perte au séchage (LOD) peut être une méthode alternative, devant être corrélée à la titration KF pour valider la mesure. L'analyseur d'humidité Sartorius Mark 3 montre une bonne corrélation avec les normes KF pour plusieurs résines plastiques, et est une méthode reconnue par la norme ASTM 6980.
Introduction :
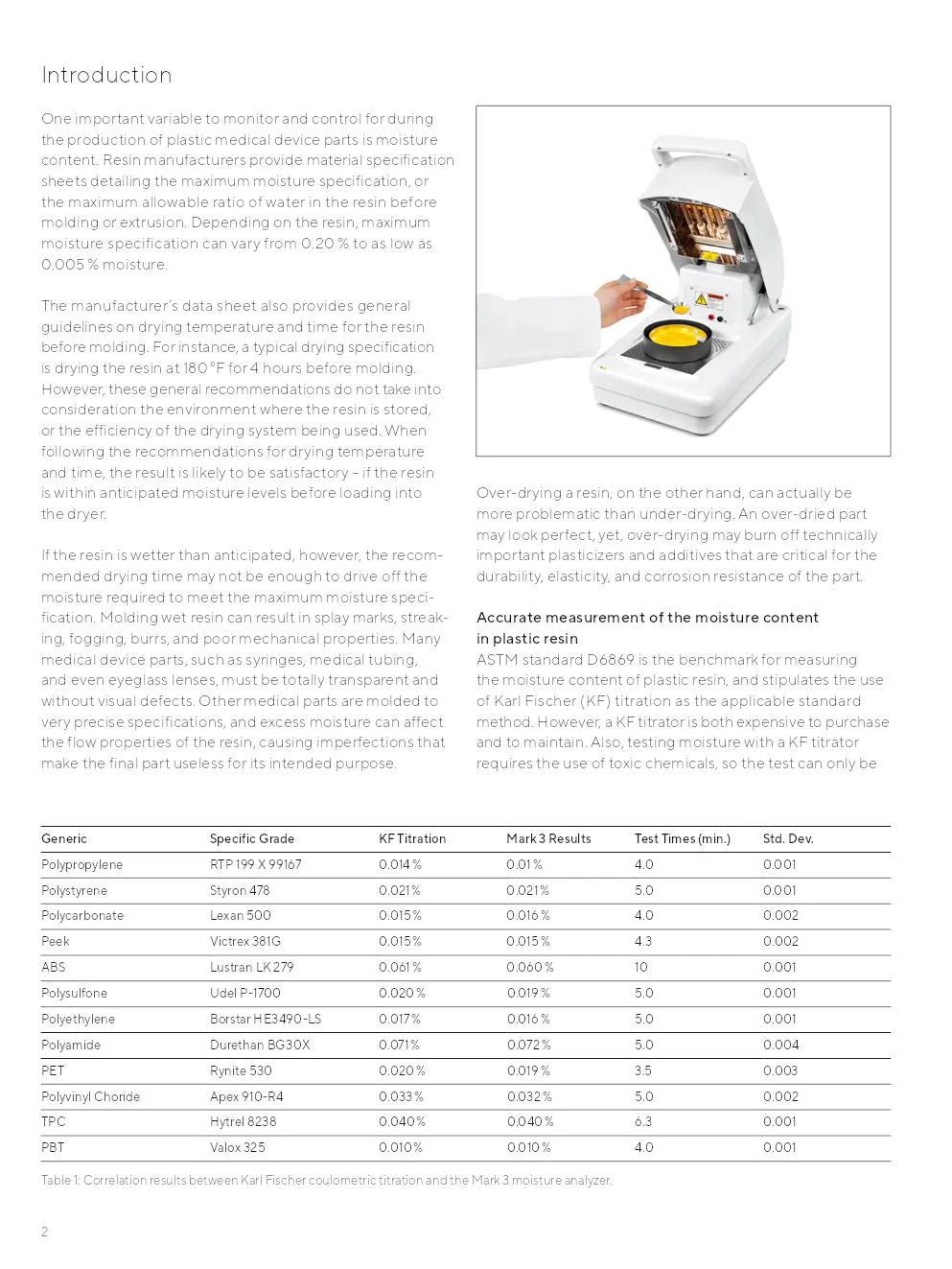
Contrôler le contenu en humidité lors de la production de pièces médicales est critique. Les fabricants fournissent des fiches de spécifications mentionnant l'humidité maximale tolérable, qui varie selon la résine. Un séchage inapproprié peut entraîner des défauts visuels et structurels. La norme ASTM D6869 est la référence pour la mesure de l'humidité, bien que coûteuse et nécessitant un traitement par des chimistes qualifiés.
Méthodes et résultats :
Le Mark 3 est configuré pour corréler ses résultats avec la norme ASTM 6980 de titration KF. Des programmes ont été développés pour plus de 7 000 résines, garantissant des résultats traçables et reproductibles. Le tableau ci-dessous présente des corrélations pour différentes résines, montrant que le Mark 3 offre des résultats comparables à la norme KF :
- Polypropylène : 0.014% (KF) vs 0.01% (Mark 3)
- Polystyrène : 0.021% (tant KF que Mark 3)
- Polycarbonate : 0.015% (KF) vs 0.016% (Mark 3)
- Peek : 0.015% (tant KF que Mark 3)
- ABS : 0.061% vs 0.060%
- Polysulfone : 0.020% vs 0.019%
- Polyéthylène : 0.017% vs 0.016%
- Polyamide : 0.071% vs 0.072%
- PET : 0.020% vs 0.019%
- Polychlorure de vinyle : 0.033% vs 0.032%
- TPC : 0.040% (tant KF que Mark 3)
- PBT : 0.010% (tant KF que Mark 3)
Conclusions :
Les fabricants de pièces médicales reconnaissent la nécessité de surveiller l'humidité des résines plastiques. L'analyseur d'humidité Mark 3 est un bon investissement, offrant des coûts de maintenance faibles et réduisant les risques de pièces rejetées. Pour plus d'informations, visitez www.sartorius.com.
Entreprises concernées :
Document protégé
Document uniquement accessible aux visiteurs connectés
Pas encore de compte ?
Inscrivez-vous
Déjà un compte ? Cliquez ici pour vous connecter
Connectez-vous