guide 2022 sur le contrôle qualité et les tests des dispositifs médicaux
Guide sur le contrôle qualité et tests des dispositifs médicaux

Contenu du document
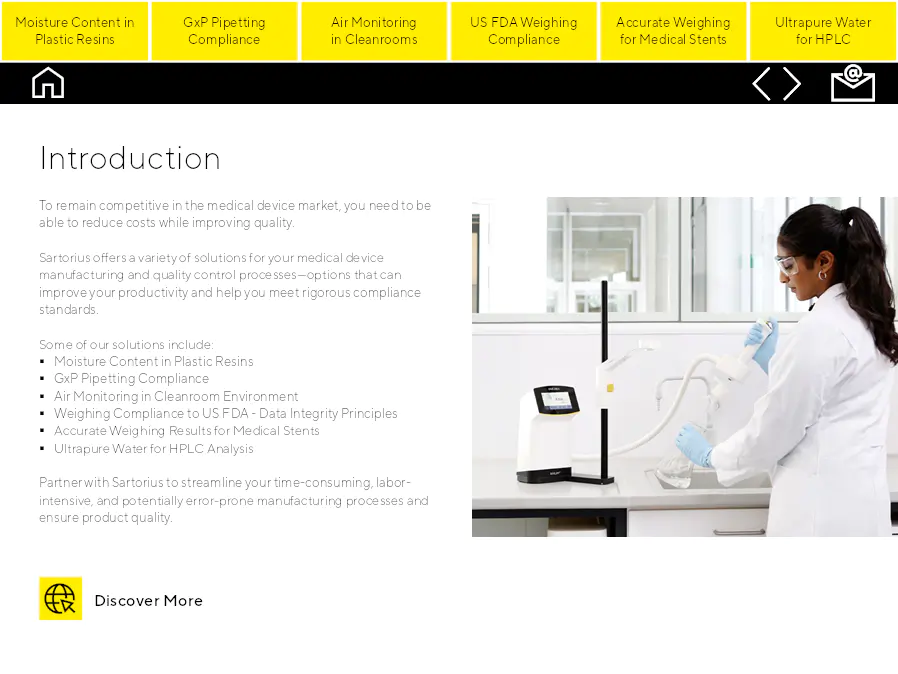
Introduction
Pour rester compétitif sur le marché des dispositifs médicaux, vous devez être capable de réduire les coûts tout en améliorant la qualité. Sartorius propose diverses solutions pour vos processus de fabrication et de contrôle qualité des dispositifs médicaux, des options qui peuvent améliorer votre productivité et vous aider à respecter des normes de conformité rigoureuses.
- Tenue de l'humidité dans les résines plastiques
- Conformité GxP pour le pipetage
- Surveillance de l'air dans les environnements de salles blanches
- Conformité de la pesée selon les principes d'intégrité des données de la FDA américaine
- Résultats de pesée précis pour les stents médicaux
- Eau ultrapure pour l'analyse HPLC
Partenariat avec Sartorius pour rationaliser vos processus de fabrication chronophages, laborieux et potentiellement sources d'erreurs et garantir la qualité des produits.
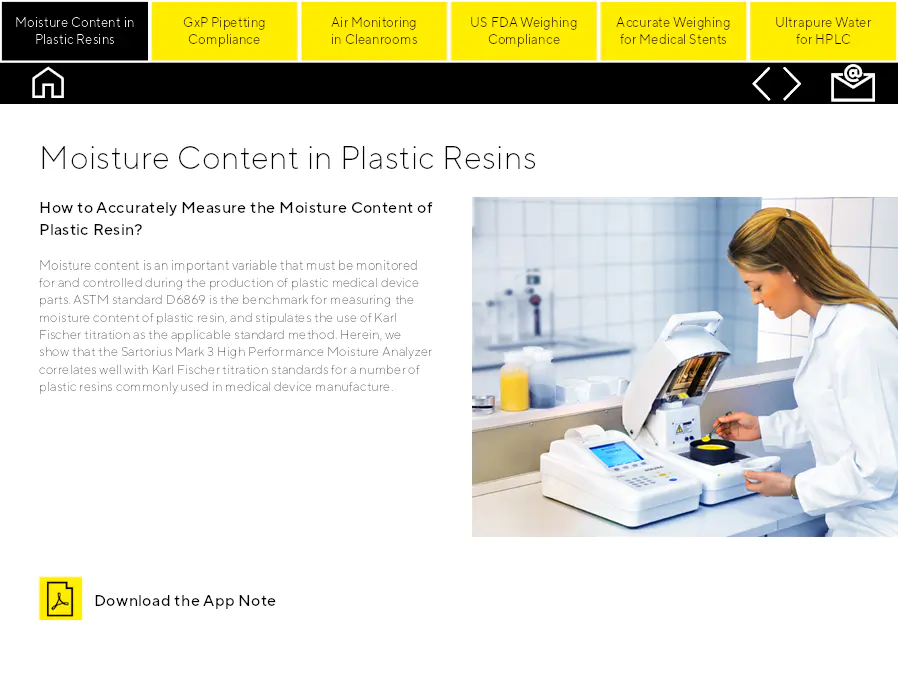
Moisture Content in Plastic Resins
Comment mesurer avec précision l'humidité des résines plastiques ? L'humidité est une variable importante qui doit être surveillée et contrôlée lors de la production de pièces en plastique pour dispositifs médicaux. La norme ASTM D6869 est le standard de référence pour mesurer l'humidité des résines plastiques et prescrit l'utilisation de la titration Karl Fischer comme méthode standard applicable. Nous montrons ici que l'analyseur d'humidité haute performance Sartorius Mark 3 est bien corrélé avec les normes de titration Karl Fischer pour un certain nombre de résines plastiques couramment utilisées dans la fabrication de dispositifs médicaux.
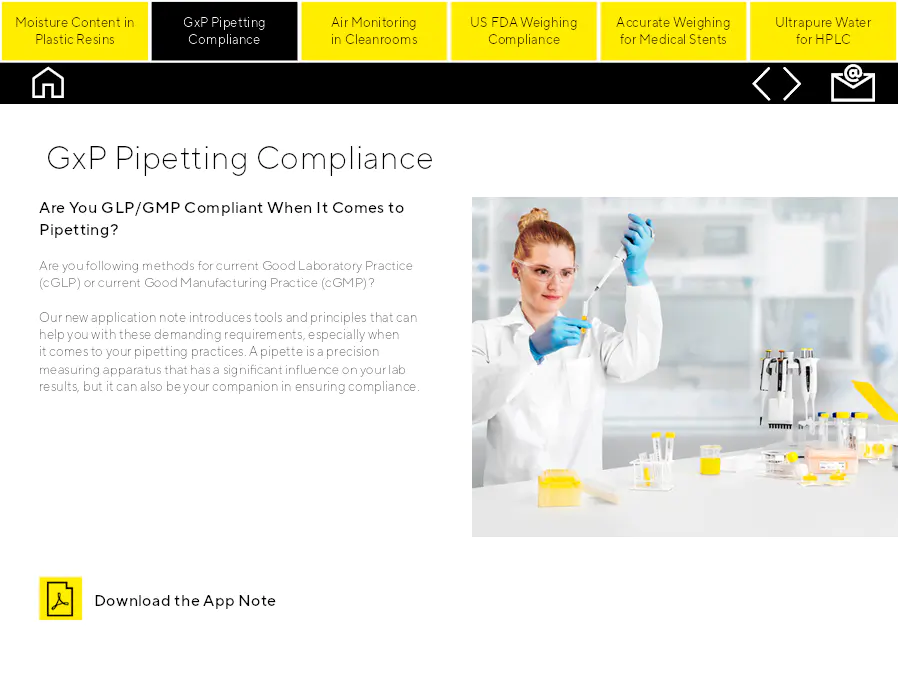
GxP Pipetting Compliance
Êtes-vous conforme aux GLP/GMP en ce qui concerne le pipetage ? Suivez-vous les méthodes des pratiques de bonnes pratiques de laboratoire actuelles (cGLP) ou des bonnes pratiques de fabrication actuelles (cGMP) ? Notre nouvelle note d'application introduit des outils et des principes qui peuvent vous aider avec ces exigences exigeantes, en particulier en ce qui concerne vos pratiques de pipetage. Une pipette est un appareil de mesure de précision qui a une influence significative sur vos résultats de laboratoire, mais elle peut également être votre compagnon pour garantir la conformité.
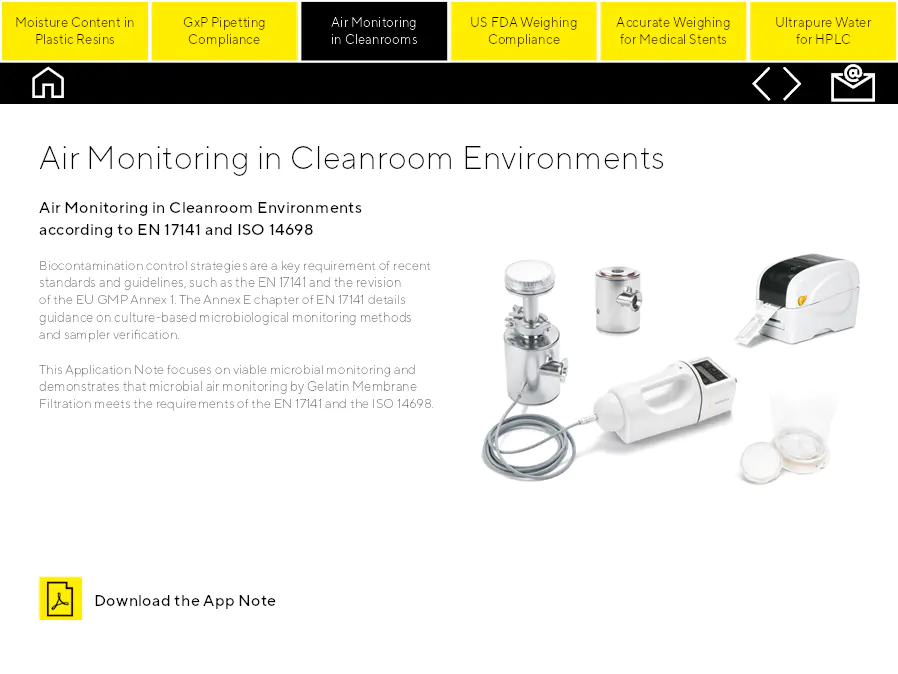
Air Monitoring in Cleanroom Environments
Surveillance de l'air selon les normes EN 17141 et ISO 14698. Les stratégies de contrôle de la biocontamination sont une exigence clé des normes et directives récentes, telles que l'EN 17141 et la révision de l'annexe 1 de l'UE GMP. Le chapitre Annex E de l'EN 17141 détaille les conseils sur les méthodes de surveillance microbiologique basées sur la culture et la vérification de l'échantillonneur. Cette Note d'application se concentre sur la surveillance microbienne viable et démontre que la surveillance de l'air microbien par filtration sur membrane de gélatine répond aux exigences des normes EN 17141 et ISO 14698.
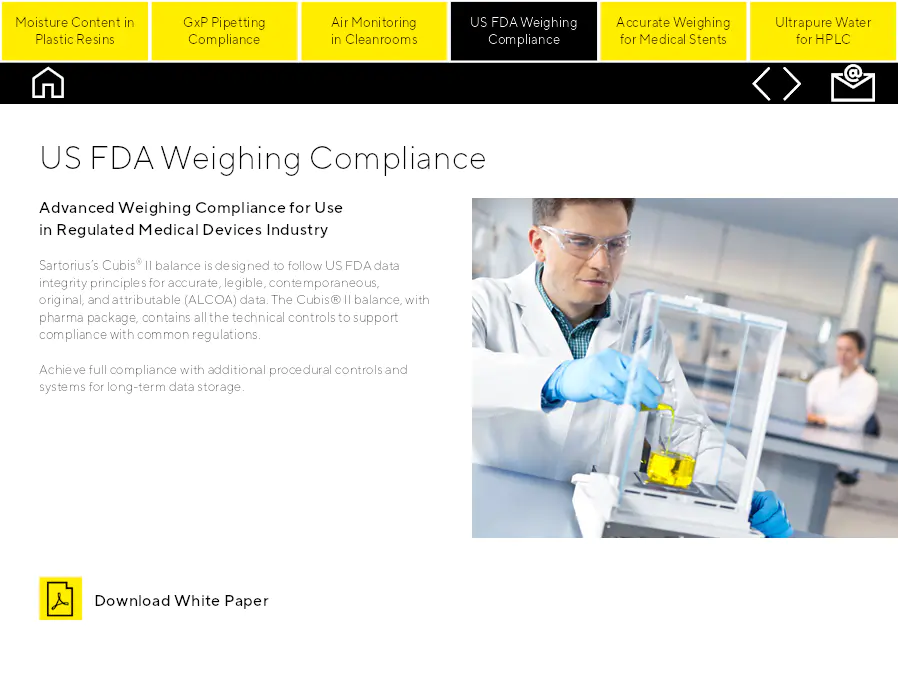
US FDA Weighing Compliance
Conformité avancée de la pesée pour utilisation dans l'industrie des dispositifs médicaux réglementée. La balance Cubis® II de Sartorius est conçue pour suivre les principes d'intégrité des données de la FDA des États-Unis pour des données précises, lisibles, contemporaines, originales et attribuables (ALCOA). La balance Cubis® II, avec package pharma, contient tous les contrôles techniques pour soutenir la conformité avec les réglementations communes. Atteignez la pleine conformité avec des contrôles procéduraux supplémentaires et des systèmes pour le stockage à long terme des données.
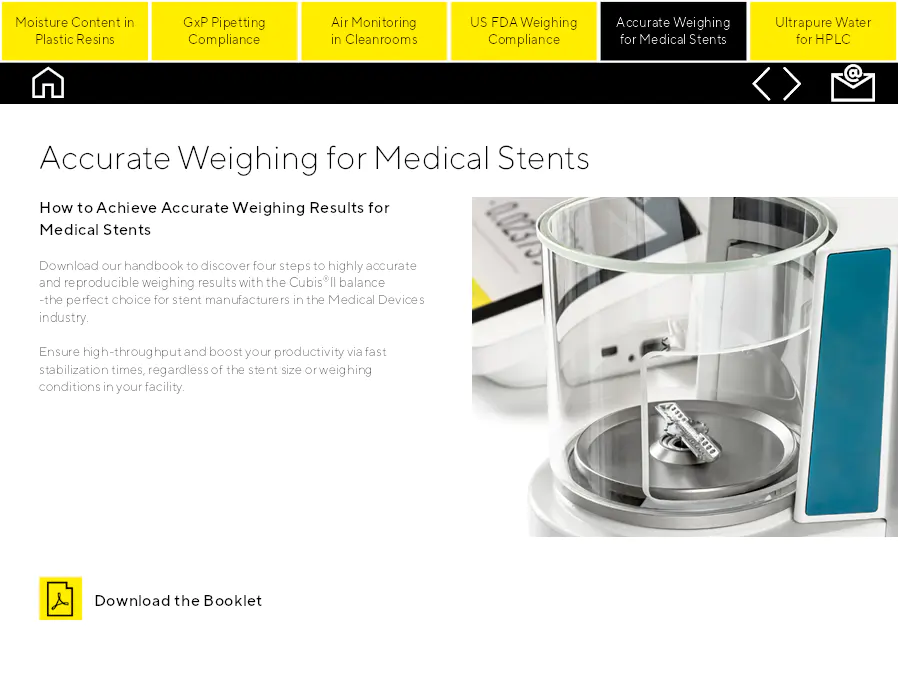
Accurate Weighing for Medical Stents
Comment obtenir des résultats de pesée précis pour les stents médicaux. Téléchargez notre manuel pour découvrir quatre étapes pour des résultats de pesée très précis et reproductibles avec la balance Cubis®II - le choix parfait pour les fabricants de stents dans l'industrie des dispositifs médicaux. Assurez un haut débit et augmentez votre productivité grâce à des temps de stabilisation rapides, quelles que soient la taille du stent ou les conditions de pesée dans votre installation.
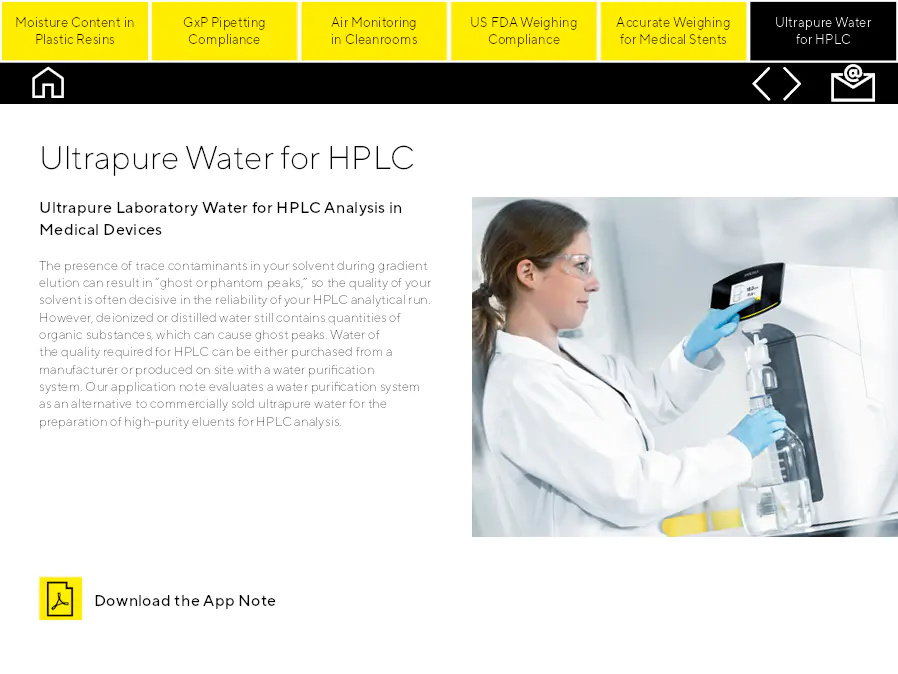
Ultrapure Water for HPLC
Eau de laboratoire ultrapure pour l'analyse HPLC dans les dispositifs médicaux. La présence de contaminants traces dans votre solvant pendant l'élution par gradient peut entraîner des pics dits 'fantômes', donc la qualité de votre solvant est souvent décisive pour la fiabilité de votre analyse HPLC. Cependant, l'eau déionisée ou distillée contient encore des quantités de substances organiques, ce qui peut provoquer des pics fantômes. L'eau de la qualité requise pour HPLC peut être achetée auprès d'un fabricant ou produite sur place avec un système de purification de l'eau. Notre note d'application évalue un système de purification de l'eau comme alternative à l'eau ultrapure commercialement vendue pour la préparation d'élusants de haute pureté pour l'analyse HPLC.
Entreprise(s) concernée(s) :
Document protégé
Document uniquement accessible aux visiteurs connectés
Pas encore de compte ?
Inscrivez-vous
Déjà un compte ? Cliquez ici pour vous connecter
Connectez-vous