application note sur le MD8 Airscan pour la surveillance microbienne continue en salle blanche
note d'application sur la surveillance continue de l'air dans les environnements de salle blanche

Contenu du document
Introduction
La surveillance environnementale est une partie importante de l'assurance qualité pour les environnements de production de produits pharmaceutiques stériles. Surtout pour les lignes de remplissage aseptique où les produits sont remplis sans étape de stérilisation terminale, il est crucial pour la sécurité des produits et constitue une partie essentielle de la stratégie de contrôle qualité. Ces environnements de fabrication classés ISO 5 doivent avoir moins de 1 unité formant colonie (CFU) par mètre cube d'air.
Une méthode typique pour surveiller la contamination de l'air consiste à aspirer activement l'air et à le filtrer à travers des filtres en gélatine spéciaux. Selon l'annexe 1 du guide EU GMP, un volume d'échantillon minimum de 1 mètre cube d'air doit être prélevé par point d'échantillonnage. Considérant un quart de travail de 8 heures, 1 mètre cube est un volume d'échantillon trop faible pour juger de manière fiable la qualité de l'air de l'environnement de fabrication. Une approche pour améliorer la sécurité des produits serait la mise en œuvre d'une surveillance continue de l'air couvrant l'ensemble du processus de production (à plusieurs points d'échantillonnage).
Contrairement aux plaques d'agar, qui se dessécheraient lors d'un échantillonnage à long terme, les filtres à membrane de gélatine peuvent être utilisés pendant toute la période de 8 heures. L'intervention humaine, telle que le changement des plaques d'agar, pourrait ainsi être évitée, réduisant ainsi le risque de contaminations secondaires à presque zéro.
Matériaux et méthodes
La viabilité des micro-organismes sur les filtres en gélatine a été examinée pendant la filtration à long terme de l'air filtré. Le terme "air filtré" décrit l'air ISO 5 filtré HEPA de l'enceinte de sécurité biologique de classe 2 utilisée.
Les filtres en gélatine de test et de référence ont d'abord été exposés à de l'air non stérile pendant 30 minutes. Les échantillonneurs d'air MD8 Airscan® (réglés à un débit d'air de 2,0 m3/h) ont été placés dans un environnement de laboratoire non contrôlé à environ 30–40 cm l'un de l'autre. Cet emplacement a été choisi pour créer des conditions environnementales spéciales. Là, une humidité relative plus élevée (~ 57 ± 6?% et température : ~ 21 ± 1?°C) était attendue.
Ensuite, les filtres de test ont été utilisés pour échantillonner de l'air filtré pendant une période supplémentaire de 8 heures. Pour la filtration de l'air ISO 5, les têtes d'échantillonnage MD8 Airscan® ont été placées sous une hotte à flux laminaire.
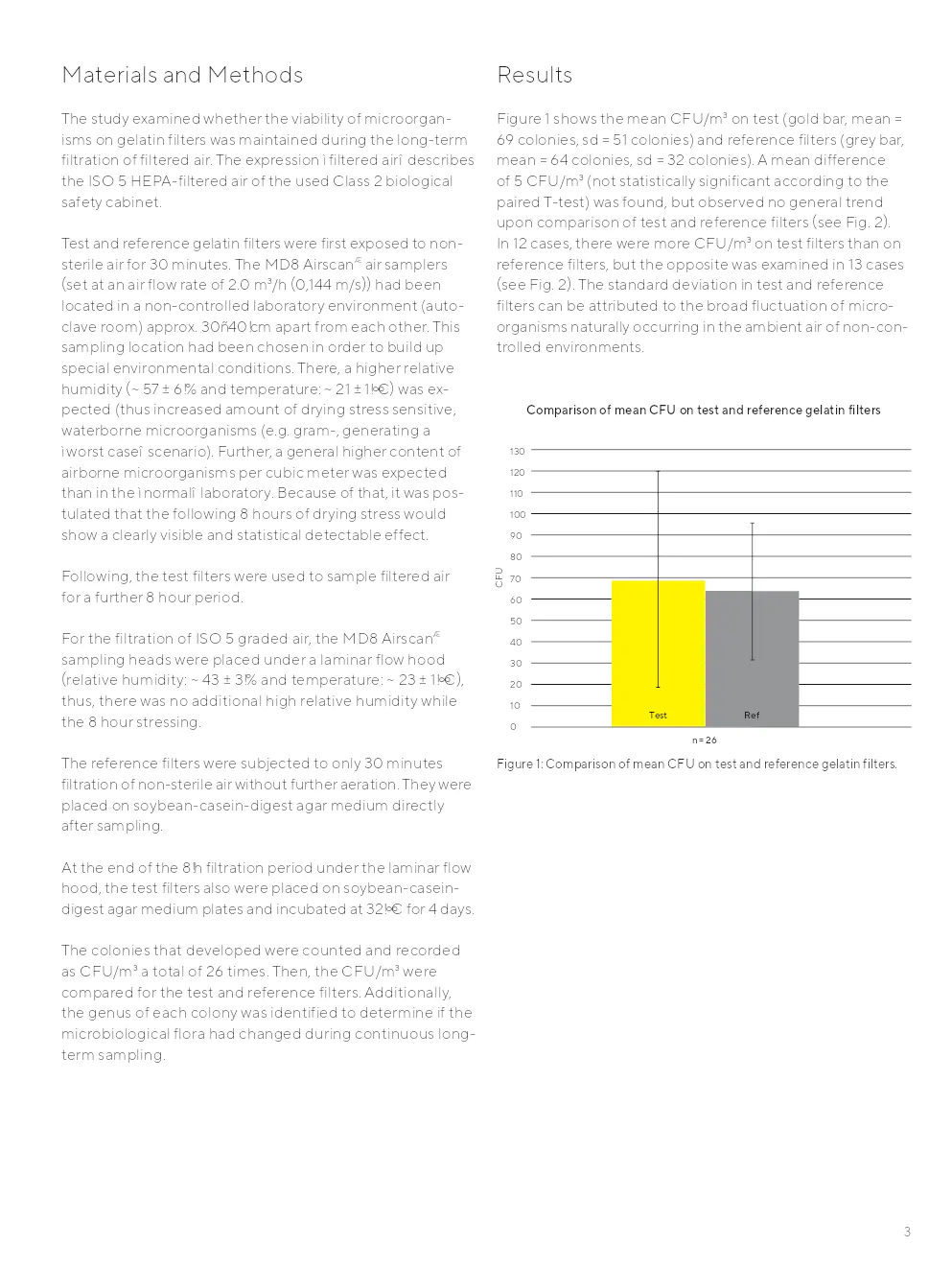
Résultats
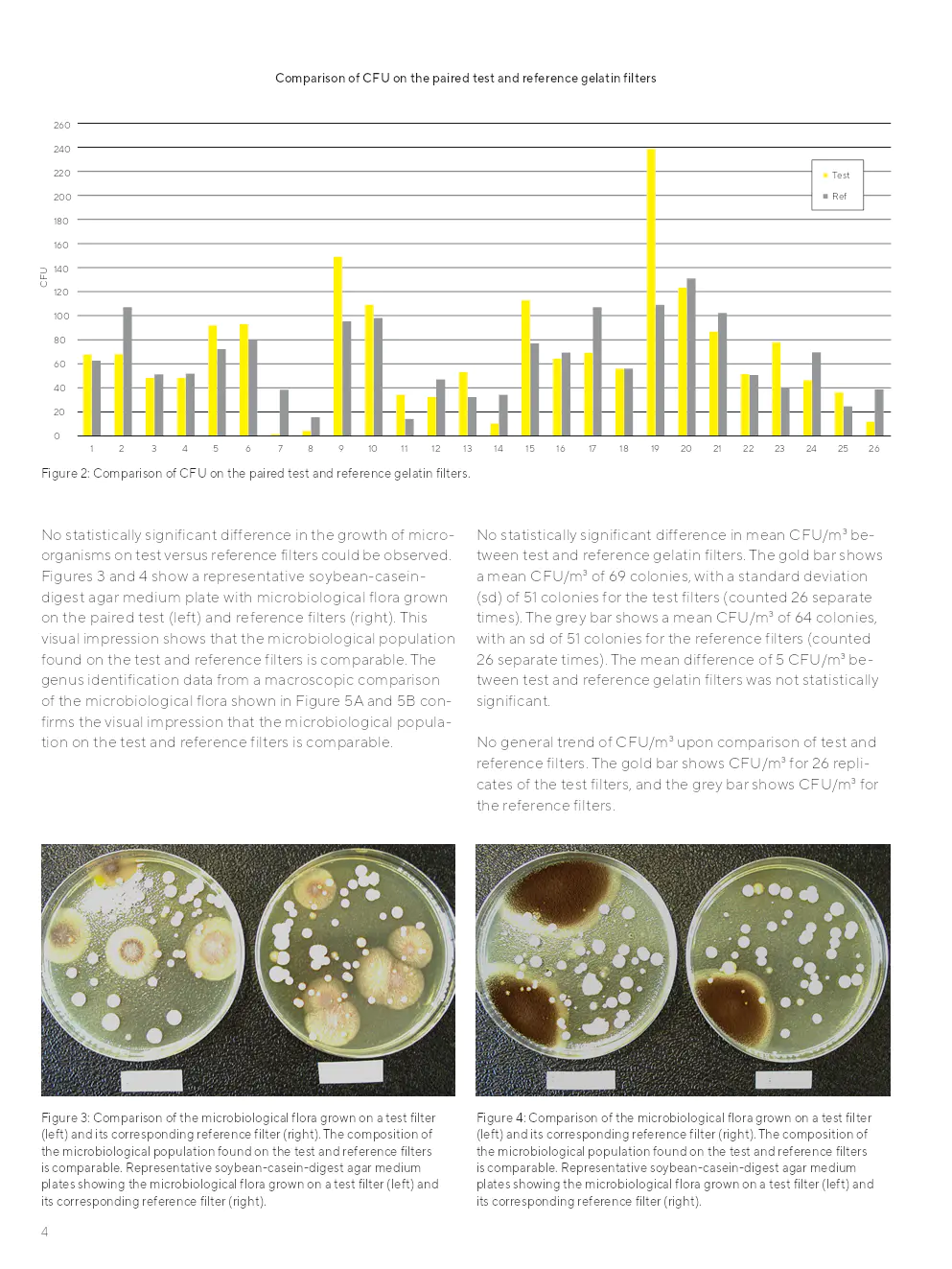
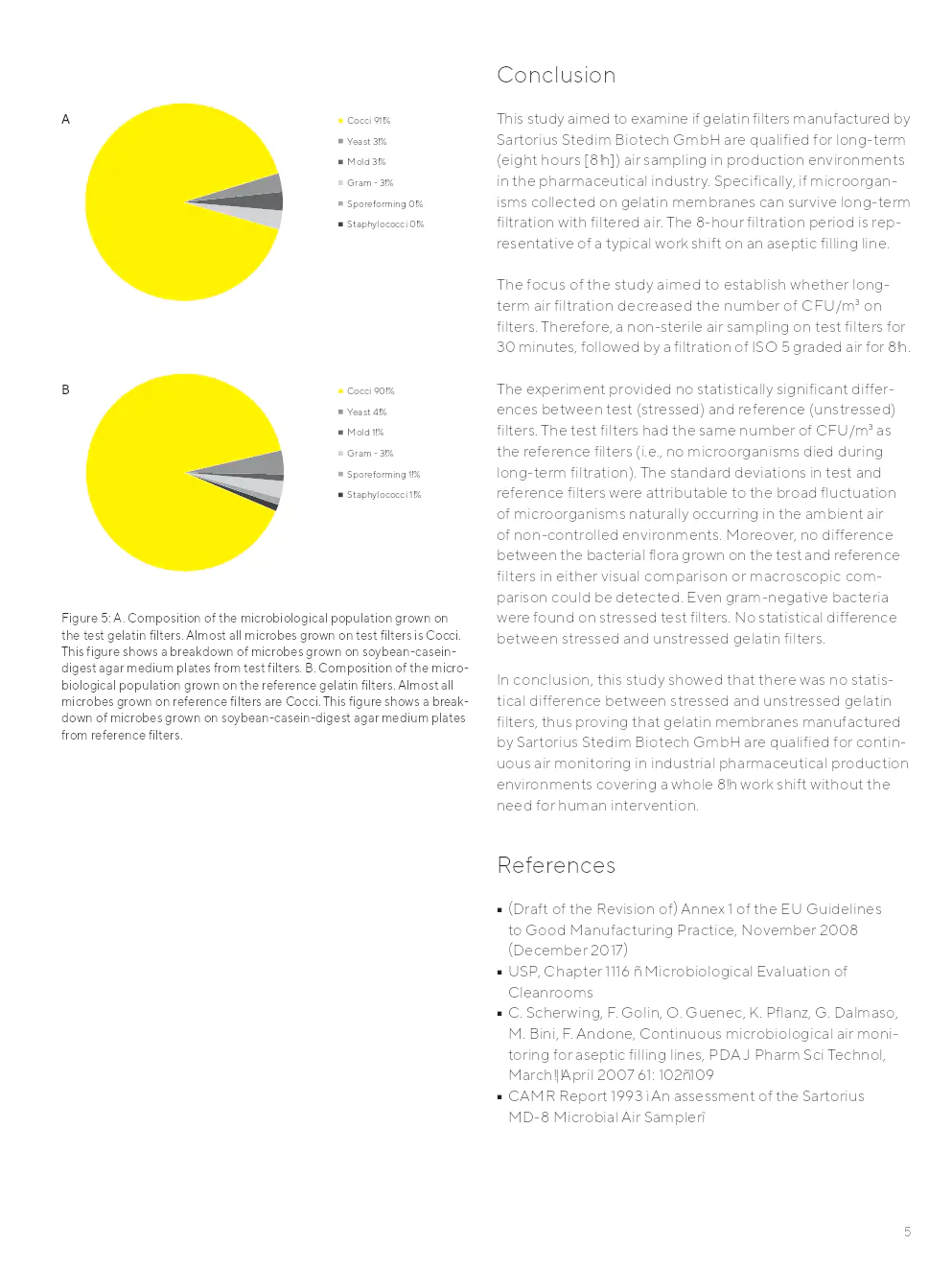
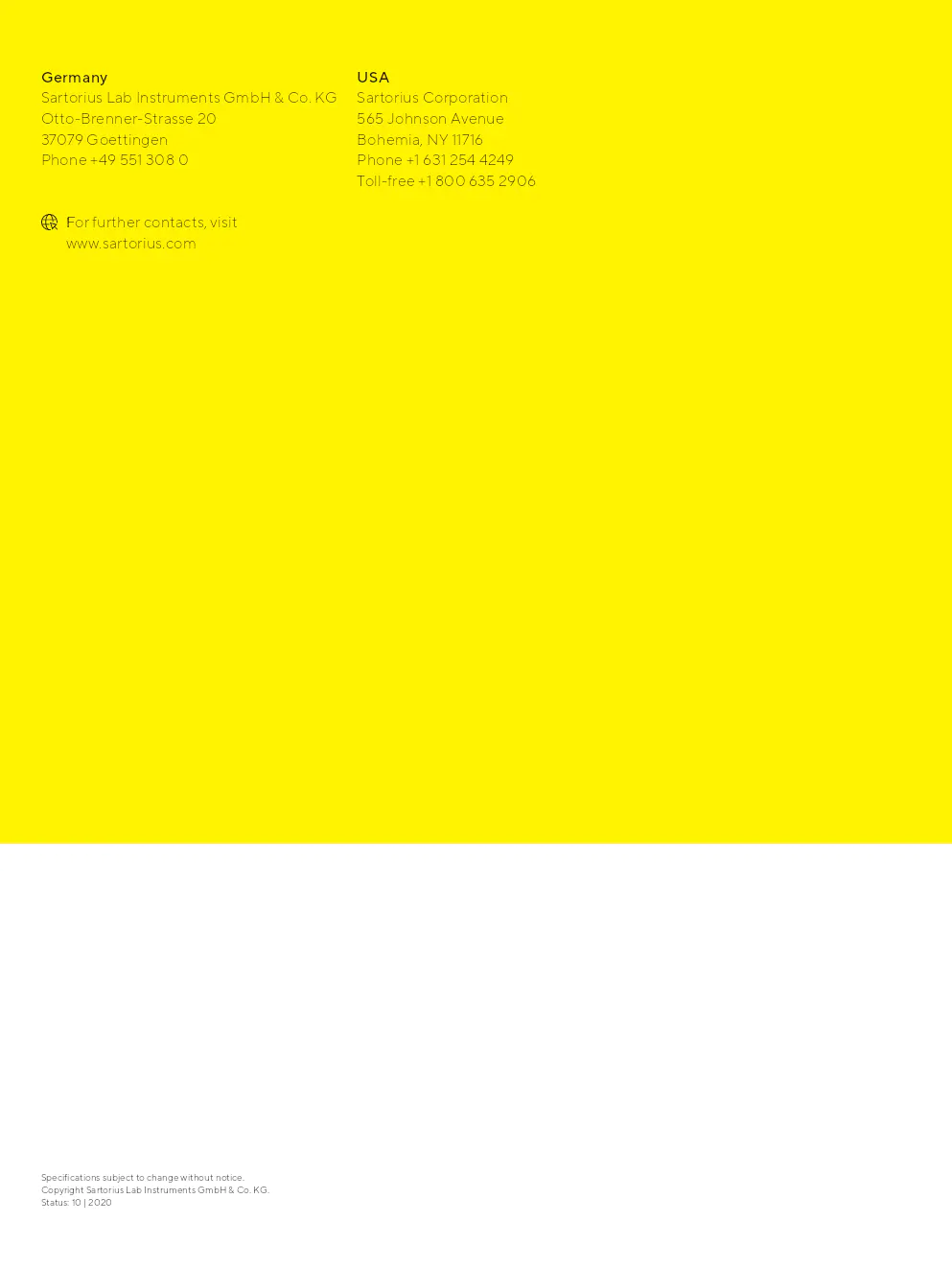
Aucune différence statistiquement significative dans la croissance des micro-organismes sur les filtres de test par rapport aux filtres de référence n'a été observée. Les populations microbiennes trouvées sur les filtres de test et de référence étaient comparables.
Conclusion
Cette étude visait à déterminer si les filtres en gélatine fabriqués par Sartorius Stedim Biotech GmbH sont qualifiés pour l'échantillonnage d'air à long terme (huit heures) dans les environnements de production de l'industrie pharmaceutique. Les tests ont montré qu'il n'y avait pas de différence statistiquement significative entre les filtres de test (stressés) et de référence (non stressés).
Entreprises concernées :
Document protégé
Document uniquement accessible aux visiteurs connectés
Pas encore de compte ?
Inscrivez-vous
Déjà un compte ? Cliquez ici pour vous connecter
Connectez-vous